Effective 11/1/2016 west coast operations have merged with Cochrane and Company
Ph. (619) 462-1128
Contact Valerie Valentine
Washington, DC Branch
2001 N. Lincoln Street
Arlington, VA 22207
Ph. (703) 248-2566
Fax (703) 248-2565
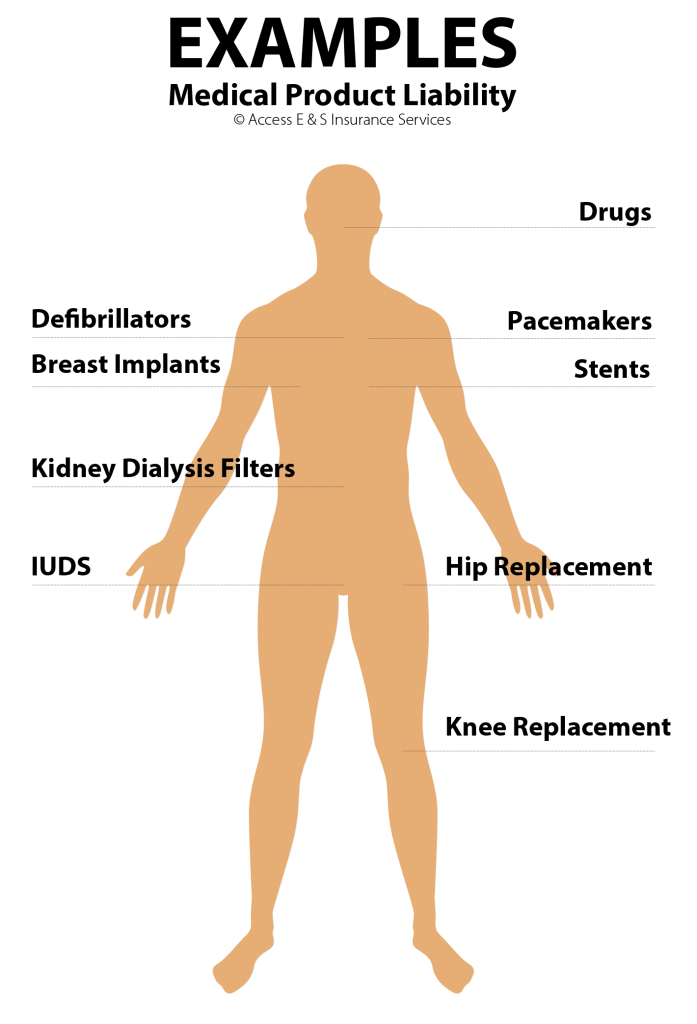
Is Medical Product Liability Insurance Right For Your Clients?
Do you work with any medical device manufacturers or distributors? Or maybe you have talked to someone who has developed a new product for the medical field. They need Medical Product Liability insurance.
Why, you ask? They’re legally on the hook for any damage caused by their products – period. All it takes is one product to go south to put their entire operation in serious jeopardy.
What Does Medical Product Liability Insurance Guard Against?
- Defectively Manufactured Devices: An unsafe defect in the production process, or even in shipping.
- Defectively Designed Devices: The product design is intrinsically unsafe.
- Defectively Marketed Devices: Instructions, warnings, recommendations, or even ads were insufficient or misleading regarding the end user’s risk.
Sometimes, a medical device will be on the market for a long time before causing serious injuries, typically because the device breaks down in some way. In some cases, the victims may claim that the manufacturer knew of the danger but deliberately concealed it or delayed taking the product off the market.
Lawsuits making any of these claims may seek awards for medical costs, compensatory damages, economic or business damages, attorneys’ fees, and punitive damages. No matter how conscientiously one can plan, their business faces risk exposure every step of the way. Once can never remove all risk, but it can be managed with adequate coverage.
A key point is that a business can be held responsible for any products they distribute, whether they manufactured them or not. In fact, as part of a “stream of commerce” a company can be found negligent as part of the liability model followed by most state courts. In other words, if a firm places the product into the commerce stream, they may be held legally liable for any harm to the end user.
What Do Medical Product Liability Policies Cover:
Medical product Liability policies cover medical costs, legal fees and awarded damages. It can potentially be extended to include the cost of recalling and destroying products as well as the resulting loss of business.
How Medical Malpractice Differs From Product Liability in Law
Generally speaking, medical devices have been treated like all other consumer products with regard to both negligence and strict liability in most jurisdictions.
However, there is interaction between product liability and medical practice, and that interaction has consequences for medical device producers. Fear of malpractice claims has encouraged what is known as “defensive medicine.” This is when the practitioner exercises extreme caution by ordering a battery of tests that may not be medically necessary in order to protect themself against future claims. This behavior has led to overuse of some medical devices potentially leading to patient harm.
When are Medical Product Liability Files Claimed?
Malpractice and product liability cases often are filed simultaneously. For example, in many IUD cases, women sue both their doctors and the product manufacturer. The manufacturer’s liability is based on failure to warn of dangerous side effects or on production of a defectively designed product. A doctor’s failure to inform the patient of risks associated with IUD use, failure to perform a thorough examination, negligent insertion or removal of an IUD, failure to warn of the risks of pregnancy when the device is in place, and failure to monitor the patient for adverse reactions can all establish claims.
Next Steps…
Access E&S can assist you because we offer a wide range of product liability risks such as Medical Product Liability. Contact us to find the right product liability coverage for your clients!